Patients
Elevate the palate. Alleviate snoring.1,2,3
What is the Elevo Snoring Invention Set?
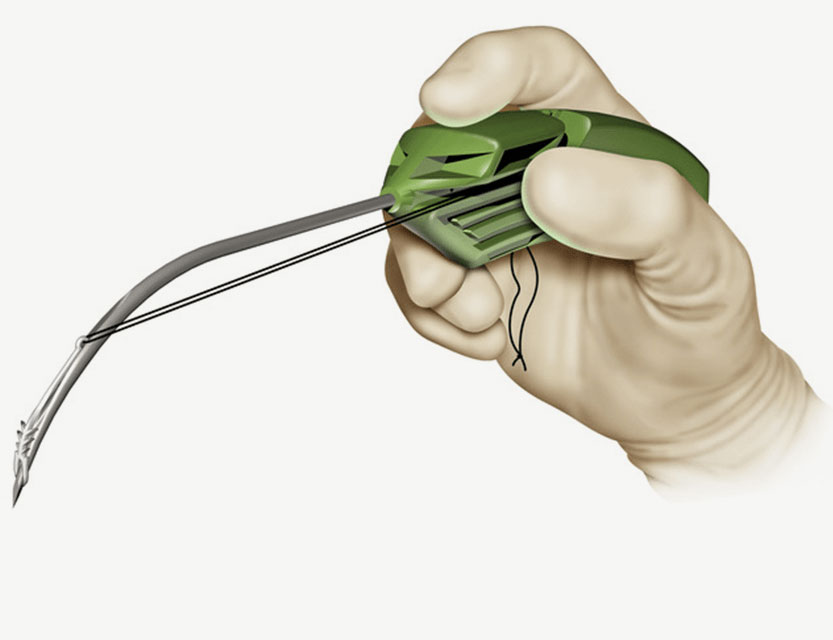
Created by Zelegent, Inc. and distributed by Cook Medical, the Elevo Snoring Intervention Set is a product designed to enhance patient treatment for snoring. The set is used to perform Elevoplasty®, a procedure that provides lift and stiffening to the soft palate through the placement of three bi- directional, barbed resorbable suture implantsa.
The concept of the set was developed by Drs. Yosef P. Krespi and David O. Volpi. Their goal was to develop a minimally invasive procedure which could be performed by sleep specialty otolaryngologists to treat snoring at the palatal level without the need for invasive surgery.

The Elevo Snoring Intervention Set is intended for use in stiffening the soft palate tissue, which may reduce the severity of snoring in some individuals. It is indicated for symptomatic, habitual, and social snoring due to palatal flutter.3
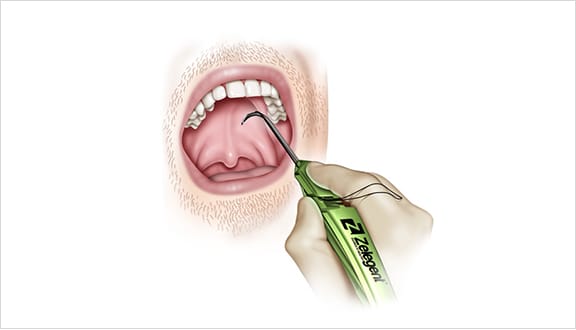
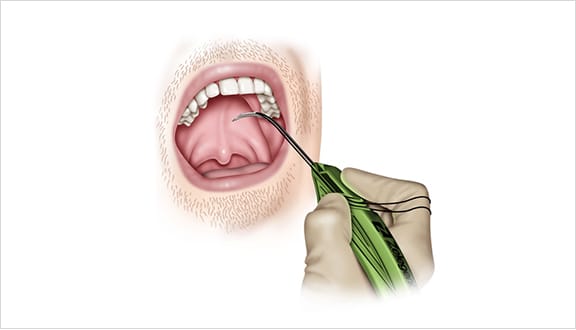
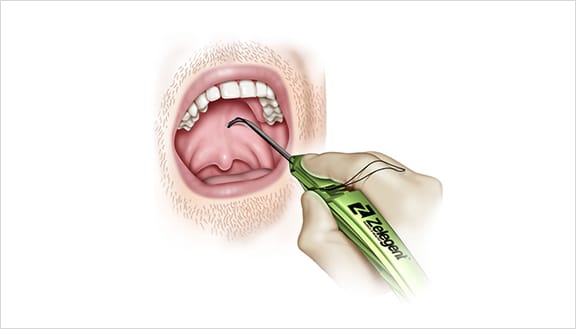
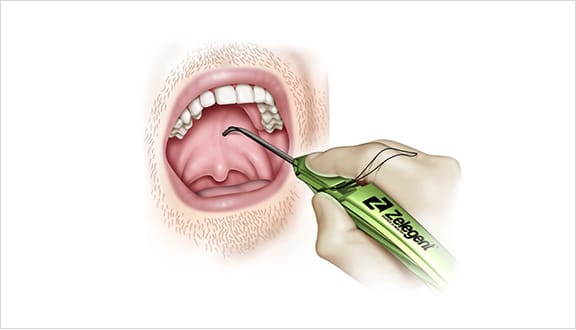
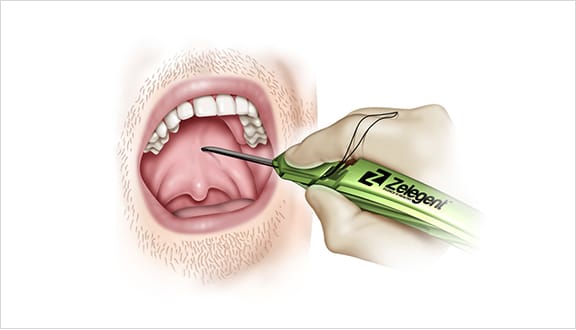
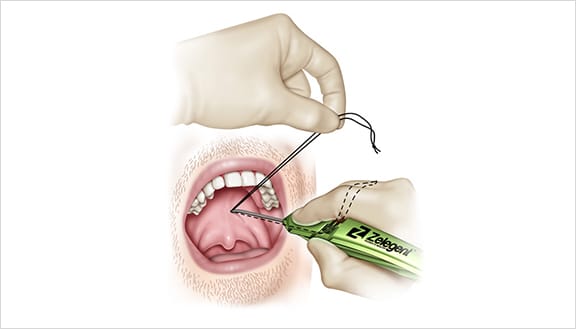
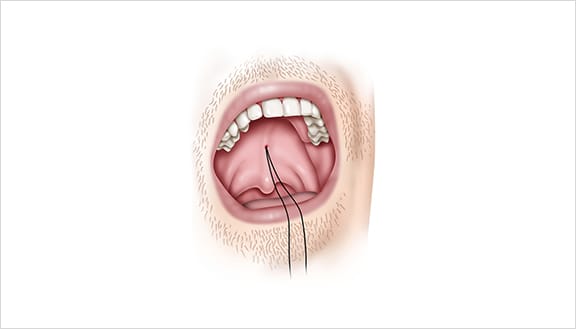
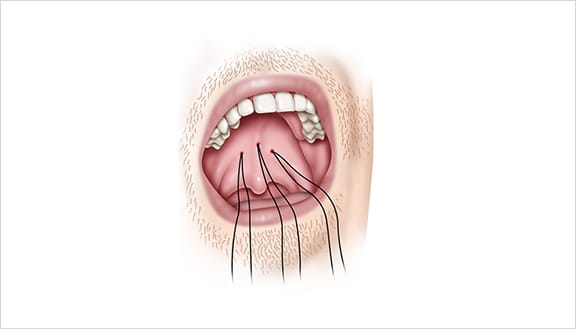
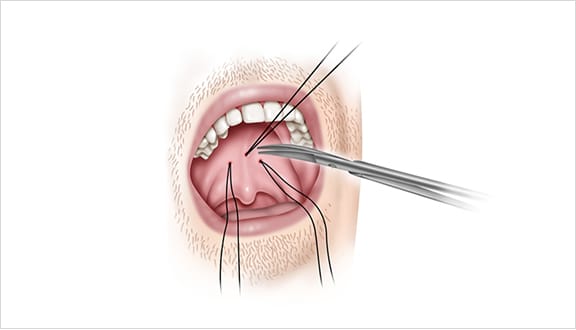
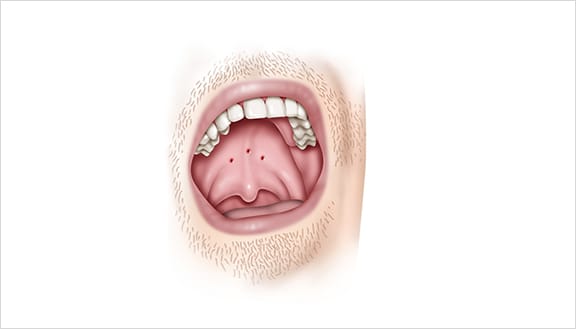
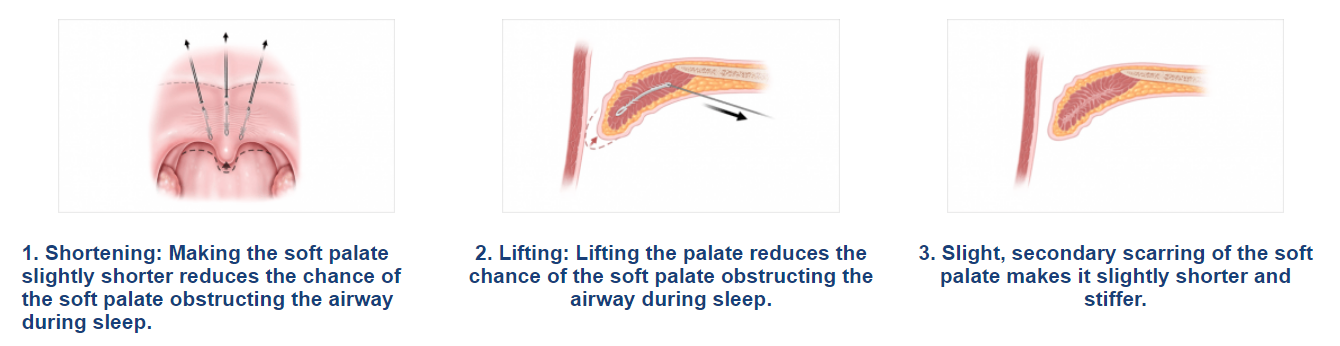
The Elevoplasty procedure provides lift and stiffening to the soft palate through the placement of three barbed, resorbable sutures.
Elevoplasty Procedure
Elevo’s delivery system allows the placement of bi-directional, barbed resorbable suture implants that provide lift to the soft palate without surgery, thereby alleviating snoring by three mechanisms.
This provides lift to the soft palate without surgery, thereby alleviating snoring by three mechanisms.
30%
reduction in sVAS
at 180 days post -procedure
As the suture implants are resorbed over time, the soft palate is stiffened. Due to this lifting and stiffening action created in the soft palate, patients experienced a 30.3% reduction in sVAS at 180 days post-procedure. 1,2
And, Patients experienced significant and prolonged improvement of sleep quality and daytime alertness
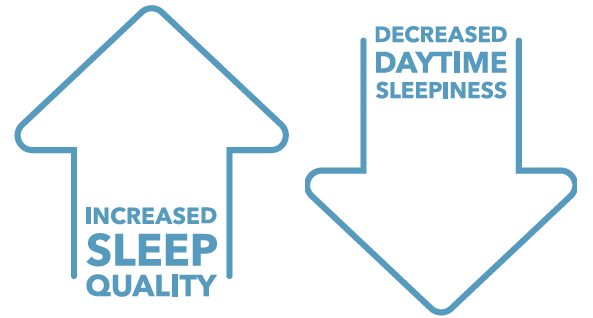
Sleep outcome measurements resulting from the S.I.LE.N.C.E. Clinical Trial 1,2
Epworth Sleepiness ScaleThe mean score decreased from 6.63 at baseline to 4.63 at 180 days post-procedure (p<.01).
Pittsburgh Sleep Quality IndexThe mean score decreased from 7.04 at baseline to 5.51 at 180 days post-procedure (p<.001).
SNAP home sleep testThe snoring loudness ratio measurements decreased, though this was not deemed statistically significant.
Elevoplasty® Procedural Techniques
Elevoplasty® is a simple, minimally-invasive procedure designed to be performed in an office setting in approximately ten to fifteen minutes.
Physician training can be scheduled by Contacting Us.
Elevoplasty Procedural Animation
Elevoplasty Procedural Animation
Elevoplasty Physician Brochure

Physician Website – Elevoplasty Educational Support Kit

1 Elevo kit snoring intervention device. Premarket notification 510(k) K181107. 2 Friedman et al. A new office-based procedure for treatment of snoring – The S.I.Le.N.C.E study. Laryngoscope Investigative Otolaryngology. 2020;5:24–30.
3 Indications for Use: The Elevo® Kit Snoring Intervention Device is intended for use in stiffening the soft palate tissue; which may reduce the severity of snoring in some individuals.